 |
 |
 |
 |
|
Research Highlights |
|
 |
|
17
Sept 2014

McQuibban and McNeill labs reveal a novel signaling pathway in latest issue of Cell
Planar cell polarity (PCP), is a form of tissue organization that is critical for normal development. Dysfunction in PCP can result in several human cancers. PCP can be readily assessed in the Drosophila eye, by the ordered ommatidial array shown below:
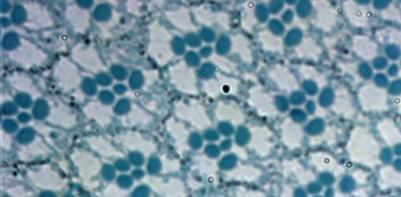
One of the key factors in the PCP pathway is the cell adhesion molecule called Fat. Using a combination of genetics and biochemistry, the McNeill (Mt. Sinai, Dept. Mol. Gen.) and McQuibban labs have discovered that Fat undergoes a proteolytic cleavage that releases a soluble cytoplasmic fragment. This cleavage unmasks an embedded mitochondrial targeting motif and results in Fat import into mitochondria. Once inside mitochondria, Fat regulates changes in intracellular metabolism. This is the first example of a cell surface protein being relocated to mitochondria to orchestrate an important signaling pathway
Read more about Angus' research on the McQuibban Lab website
|
|

|
|
|
|
9
May 2014

Alex Palazzo on Junk DNA
Dr. Palazzo in collaboration with T. Ryan Gregory from the University of Guelph published a perspective on “The Case for Junk DNA” that appeared in the May issue of PLoS Genetics and is highlighted in on the National Geographic website.
To read more about Alex's work, visit his lab website at:
http://biochemistry.utoronto.ca/palazzo/index.htm
|
|

|
|
|
|
12
October 2012

Trevor Moraes awarded Tier 2 Canada Research Chair
Research in the Moraes lab examines protein and ion translocation across bacterial membranes. In particular, Dr. Moraes dissects the components of these pathways examining their interactions in molecular detail. Biochemical tools such as X-ray crystallography provide atomic resolution 3D-models of the proteins, while surface plasmon resonance, isothermal calorimetry or bio-layer interferometry are used to determine kinetic parameters and validate models that describe the mechanism of action. These membrane proteins function together as a complexes to facilitate efficient transport that is essential for pathogenic bacteria to survive within the host, thus this research provides the basis for developing novel antimicrobial therapeutics.
Please visit Trevor's website for more information
|
|

|
|
|
|
20
September 2012

Chair Reinhart Reithmeier Elected Fellow of the Canadian Academy of Health Sciences
At a ceremony held in Ottawa on September 20, 2012, Biochemistry Chair Reinhart Reithmeier was admitted as a Fellow to the Canadian Academy of Health Sciences (CAHS) “having demonstrated both distinctive accomplishments and the commitment to advance health sciences”.
A major goal of the CAHS is to provide timely, informed, science-based and unbiased assessments of urgent issues affecting the health of Canadians. Reinhart, well-known internationally for his work on membrane proteins and human disease, has also been relentless in promoting the importance of basic research and the need for increases in funding to support excellence and to remain competitive.
CAHS President Thomas Marrie presents Reinhart Reithmeier with certificate of membership as a Fellow of the Canadian Academy of Health Sciences (CAHS) |
|

|
|
|
|
09
December 2011

Special issue of the Journal
of Biomolecular NMR on the occasion of Lewis Kay’s 50th birthday
In the Editorial
introducing this special issue [J Biomol NMR (2011) vol 51], Kevin
Gardner, Anthony Mittermeier and Frans Mulder note that Lewis is
being honoured for his "innovative contributions to biomolecular
NMR spectroscopy, as well as for his role as inspirer for a large
group of young research professionals."
They further
comment that "Lewis has consistently been a pioneer in the
development of novel methods essential to increasing the size of
proteins that may be studied by NMR....His lab forged ahead with
clever biochemical tricks for incorpo- rating protonated methyl
groups in otherwise deuterated proteins, and developed methyl TROSY
experiments that permit well-resolved spectra to be obtained for
high molecular weight complexes, extending to 1 MDa."
"In addition,
the Kay lab has extensively developed and applied new methodologies
for studying low-populated states of proteins and their roles in
biology. Building on transverse relaxation dispersion experiments,
the Kay lab has introduced approaches to elucidate the three-dimensional
structures and dynamics of protein states so weakly populated that
they are ‘‘invisible’’ to standard NMR experiments
and most other biophysical and structural techniques."
|
|

|
|
|
|
03 May 2011

Daniela Rotin wins "Women
of Action" Award
Daniela Rotin
was honoured by the Israel Cancer Research Fund (IRCF) as a recipient
of the prestigious "Women of Action" award at a gala event
held at the Sheraton Hotel in Toronto on May 3, 2011.
Founded in 1975,
IRCF is one of the largest private sources of funding for cancer
research in Israel and has helped to support the early work of scientists
such as 2004 Nobel laureates Aaron Ciechanover and Avram Hershko,
discoverers of the ubiquitin proteostasis system. For many years
Daniela has served on the ICRF scientific review panel that selects
research grant award winners through a rigorous peer-review system.
Close to 2,000 grants totalling over $40M have been awarded with
funds raised by ICRF at events like the gala held in Toronto.
Congratulations
to Daniela for this important recognition.
|
|

|
|
|
|
29 April 2011

David Isenman's Science
article resolves a long-standing controvery in the complement field
In a study published
in the April 29th issue of Science, Professor David Isenman
of Biochemistry and Jean van den Elsen of the University of Bath
shed light on the complex between complement receptor 2 and its
ligand C3d, both of which are constituents of an innate immune system
of our body known as complement.
The molecular
details of the CR2-C3d interface have been mired in controversy
for the past decade. Specifically, in 2001 an X-ray crystal structure
of the complex consisting of C3d and the first two domains of CR2
was published in Science. However, from the start, that
structure was discordant with much biochemical data in the literature,
and over the years the discrepancies have only increased.
The detailed
knowledge of the receptor-ligand interface provided by the new structure
of this complex has implications as both a potential therapeutic
target, in the case of antibody-mediated autoimmune diseases, and
as something that may be exploited in enhancing the efficacy of
vaccines.
“We recognize that the goal of applying this knowledge to
autoimmune therapies and enhanced vaccine efficiency will not be
trivial. But the structural scaffold for further discoveries is
now in place,” said Isenman.
Click
here to read the full article in News @ the University of Toronto.
|
|

|
|
|
|
01 September 2010

Harry Schachter Honoured with
the Rosalind Kornfeld Award
The Society
for Glycobiology has just announced that Dr. Harry Schachter (Professor
Emeritus of Biochemistry, University of Toronto; Senior Scientist
Emeritus, Research Institute, Hospital for Sick Children, Toronto)
has been awarded the Rosalind Kornfeld Award for Lifetime Achievement
in Glycobiology.
Dr. Schachter
made many seminal contributions to glycobiology and the biochemistry
of glycan synthesis for more than four decades. Early on Dr. Schachter
was a leader in establishing many robust enzyme assays to characterize
Golgi glycosyl transfersase activities in both the N- and O-glycosylation
pathways. He defined complex enzyme acceptor specificities and revealed
the order in the N-glycan branching pathway leading to complex N-glycans
on cell surface receptors and secreted proteins. His group purified
GlcNAc-transferases I and II from biological sources, cloned and
expressed the respective genes, and then he turned his attention
to their functions in development and disease. He collaborated with
Dr. Jamie Marth to generate mice with germ line deletions. Dr. Schachter’s
group then generated similar mutants in C. elegans, which has three
GlcNAc-transferase I genes. They made the triple mutant and learned
much about N-glycan synthesis in the worm by characterizing the
affected glycans and glycoproteins by mass spectrometry with Dr.
Vern Reinhold.
Dr. Schachter’s enthusiasm, integrity and forthright style
are widely recognized and appreciated, and have made him an outstanding
collaborator, an excellent communicator of science, and a model
for young scientists. He collaborated with Dr Pamela Stanley on
one of the first glycosylation mutants of cultured cells to reveal
that Lec1 CHO cells lack GlcNAcT-I activity. He worked with Dr.
Jaak Jaeken to characterize the first Carbohydrate-Deficient Glycoprotein
Syndrome (CDG-IIa; now known as Congenital Disorders of Glycosylation)
and developed a mouse model of CGD-IIa with Dr. Jamie Marth. Most
recently, Dr. Schachter has explored the biological functions of
N-glycans in Drosophila development with Dr Gabrielle Boulianne.
This study indicates that GlcNAcT-I expression in the brain of Drosophila
has a tissue-specific role in the regulation of insulin signaling
and life span. Many others, both collaborators and competitors,
have benefited from Dr. Schachter’s wide knowledge, encouragement
and advice. He has authored more than 160 original papers and 80
scholarly reviews, and edited several books.
Dr. Schachter has also served the scientific community in a number
of ways. He was an Associate Editor for the Journal of Biological
Chemistry and Chief Editor of Glycoconjugate Journal, Convenor and
Chair of the 11th International Symposium on Glycoconjugates and
the 2nd International Symposium on Glycosyl transferases. He has
been a strong supporter of glycobiology through his teaching, mentoring
of students and research lectures at international meetings. In
recognition of his seminal and numerous scientific contributions
to glycobiology in development and disease, and his leadership in
promoting the field of glycobiology, the Society for Glycobiology
has awarded Dr. Schachter the 2010 Rosalind Kornfeld Award.
|
|

|
|
|
|
01 June 2010

Shana Kelley wins Steacie Prize
NSERC announced
today that Professor Shana Kelley is one of six 2010 NSERC E.W.R.
Steacie Memorial Fellowship recipients. Each fellow receives a research
grant of $250,000 and the university normally receives a contribution
to the fellow's salary to provide teaching and administrative relief.
Shana will use the funding to further her studies on the development
of chip-based sensors.
From the
NSERC press release...
Shana is intent on developing low-cost diagnostic technologies
to be used in developing countries. She has developed a chip-based
sensor (see September 28, 2009 listing) that can detect trace quantities
of DNA, RNA and protein analytes in samples, and that has already
been applied for early diagnosis of cancer. Shana is further developing
this technology for tuberculosis detection. This involves the development
of new nanomaterials that will enable sensitive sensors to detect
minuscule traces of the deadly tuberculosis pathogen. The current
tools used for diagnosis in the parts of the world most affected
by tuberculosis and similar diseases require relatively large samples
and are too slow to provide the level of control needed to reign
in the spread of infection.
|
|

|
|
|
|
25 May 2010

Lewis Kay elected to the Royal
Society
We were delighted
to learn that Lewis Kay has been elected to the Royal Society (UK)
for his work on NMR spectroscopy. He and his group have developed
many of the recent technical advances that have pushed the size
limit of protein complexes that can be examined by NMR spectroscopy
beyond 500 kDa. For example, methyl-TROSY was used to elucidate
the structure and aspects of the dynamics of the 670 kDa 20S proteasome
core particle. He has also developed methods for studying invisible
excited states of proteins by NMR and is applying them to furthering
our understanding of protein folding and conformational dynamics.
Lewis is only
the 5th member in the over 100 year history of the Department to
receive this very prestigious honour. Other FRS (UK) are our first
Chair, Archibald Byron Macallum, Charles Hanes, Gordon Dixon and
David MacLennan.
Congratulations
to Lewis!
|
|

|
|
|
|
18 December 2009

Igor Stagljar and co-workers
discover new regulator of the EGF receptor
Reprinted
from NEWS @ U of T, by Chris Garbutt
Researchers
at the University of Toronto and Goethe University in Germany have
discovered a protein that can inhibit the growth of cancer cells,
providing crucial clues for the future development of new drugs
to treat the disease.
The protein, called HDAC6, controls the stability of the epidermal
growth factor receptor (EGFR), a key player in cancer.
"Our teams discovered that HDAC6 acts as a molecular brake
to shut down the expression of the human EGFR," said Professor
Igor Stagljar of biochemistry and molecular genetics, one of the
lead authors of a study published in the Dec. 22 issue of the journal
Science Signaling. Professor Ivan Dikic at Goethe University was
co-lead on the study.
"Since EGFR is overactive in breast, lung, colon and pancreatic
cancers, this discovery can open new avenues for cancer treatment,"
said Stagljar, a principal investigator at U of T's Terrence Donnelly
Centre for Cellular and Biomolecular Research.
EGFR is nestled into the cell membrane on the surface of human cells
where, after it gets activated by molecules called ligands, causes
cells to divide. In several cancer cell types, the activity of this
receptor is dramatically increased, which stimulates cells to grow
rapidly and out of control. Because of its key role in driving the
proliferation of cells, EGFR is a target of several cancer drugs
currently in development, as well as several approved therapies.
To study the cellular role of EGFR in human cells, Stagljar's lab
first developed a technology called MYTH, a unique test that can
monitor interactions between membrane proteins. This technology
can reveal proteins that tightly associate with EGFR on the cell
surface. Using MYTH, the researchers identified more than 80 proteins
that interact, and presumably communicate, with the human EGFR.
Among them was a cytosolic protein, HDAC6, which they showed helps
in stabilizing EGFR in human cells.
"These findings offer fresh insight into how HDAC6 regulates
EGFR degradation and provides clues for the design of improved cancer
therapies," Stagljar said. Specifically, a carefully planned
combinatorial chemotherapy that inhibits both the EGFR receptor
and its newly identified "brake" (HDAC6) could have a
beneficial effect for treating breast, lung, colon, and pancreatic
cancers.
In the next phase of their research, Stagljar and his colleagues
plan to extend the MYTH technology to interrogate all human receptors
that regulate cell proliferation and are therefore implicated in
the onset of cancer. Such a global analysis of proteins that associate
with human cell surface receptors may provide novel avenues for
the treatment of different types of cancers.
|
|
|
|
|
|
28
September 2009

Shana Kelley and co-workers
create microchip that can detect type and severity of cancer
Reprinted
from NEWS @ U of T, by Elaine Smith
As reported
Sept. 27 in Nature Nanotechnology, the research groups
of Shana Kelly and Ted Sargent have used nanomaterials to develop
an inexpensive microchip sensitive enough to quickly determine the
type and severity of a patient's cancer so that the disease can
be detected earlier for more effective treatment. Their new device
can easily sense the signature biomarkers that indicate the presence
of cancer at the cellular level, even though these biomolecules
- genes that indicate aggressive or benign forms of the disease
and differentiate subtypes of the cancer - are generally present
only at low levels in biological samples. Analysis can be completed
in 30 minutes, a vast improvement over the existing diagnostic procedures
that generally take days.
"Today,
it takes a room filled with computers to evaluate a clinically relevant
sample of cancer biomarkers and the results aren't quickly available,"
said Shana Kelley, a professor in the Leslie Dan Faculty of Pharmacy
and the Faculty of Medicine, who was a lead investigator on the
project and a co-author on the publication. "Our
team was able to measure biomolecules on an electronic chip the
size of your fingertip and analyse the sample within half an hour.
The instrumentation required for this analysis can be contained
within a unit the size of a BlackBerry." Conventional, flat
metal electrical sensors were inadequate to sense cancer's particular
biomarkers. Instead, they designed and fabricated a chip and decorated
it with nanometre-sized wires and molecular "bait." "Uniting
DNA - the molecule of life - with speedy, miniaturized electronic
chips is an example of cross-disciplinary convergence," said
Sargent. "By working with outstanding researchers in nanomaterials,
pharmaceutical sciences, and electrical engineering, we were able
to demonstrate that controlled integration of nanomaterials provides
a major advantage in disease detection and analysis."
"We rely
on the measurement of biomarkers to detect cancer and to know if
treatments are working," said Dr. Tom Hudson, president and
scientific director of the Ontario Institute for Cancer Research.
"The discovery by Dr. Kelley and her team offers the possibility
of a faster, more cost-effective technology that could be used anywhere,
speeding up diagnosis and helping to deliver a more targeted treatment
to the patient." The
team's microchip platform has been tested on prostate cancer, as
described in a paper published in ACS Nano, and head and neck cancer
models. It could potentially be used to diagnose and assess other
cancers, as well as infectious diseases such as HIV, MRSA and H1N1
flu.
01 May 2009

Shana Kelley is Named Top 40
under 40
Dr .Shana Kelley, Professor of Biochemistry at the University of
Toronto and Director, Division of Biomolecular Sciences, Faculty
of Pharmacy, has been named to this year's Top 40 Under 40.
Canada's Top 40 Under 40 is a prestigious
national program founded and managed by the Caldwell Partners to
celebrate leaders of today and tomorrow and to honour Canadians
below the age of 40 who have achieved a significant level of success.
The program is designed to promote mentorship and professional development
by introducing these leaders to the established business community
and by promoting them as role models for young Canadians. In choosing
the recipients, the board at Caldwell Partners considers the nominees'
vision and leadership; innovation and achievement; impact; community
involvement and contribution; and growth/development strategy.
In an interview
with News @University of Toronto, Shana said that this recognition
is especially meaningful to her. "I think it's quite important
that those of us who pursue careers in academics make sure that
we maximize the tangible positive impact we make -- whether it be
through our research or teaching or whatever we pursue as faculty,"
said Kelley. "This award recognizes impact and thus I think
it encourages us to be all we can be."
|
|

Shana
Kelley |
|
|
|
May 2009

Charles Deber Wins American
Peptide Society Goodman Award
Dr. Charles M. Deber, Professor of Biochemistry at the University
of Toronto and Acting Head of the Division of Molecular Structure
and Function at the Research Institute, Hospital for Sick Children,
is the first recipient of the Goodman Award of the American Peptide
Society.
Charles is known worldwide for his seminal research on the structure
and function of membrane peptides and proteins, and in the examination
of disease states that involve misfolding of membrane proteins.
At the more fundamental level he was among the first to recognize
that peptide and protein folding in membranes had fundamental differences
from peptide and protein folding in aqueous environments. This led
to a critical re-evaluation of the structural properties in amino
acids in proteins that are in membrane environments, which has provided
new tools for the design of membrane peptides and proteins. Among
these tools, the web based TM Finder Program is a most valuable
tool, available worldwide.
As a mentor and teacher, Dr. Deber’s contributions also have
been exemplary. He teaches widely acclaimed courses from large undergraduate
courses, to advanced Chemistry courses for graduate students. For
his excellent undergraduate teaching he received the W.T. Aikins
Award from the University of Toronto. He has mentored more than
60 graduate students and postdoctoral fellows, many of who have
gone on to have outstanding careers in academia and industry. Finally,
Charles has made many outstanding contributions in service to the
peptide field
worldwide. In addition to being President of the American Peptide
Society from 1991-1993, he has been a long time Editor of Peptide
Science, on the editorial board of several international journals
in the field, and has organized or
helped organize several international conferences.
The Murray Goodman Scientific Excellence
and Mentorship Award is
well-deserved recognition for the above and many other contributions.
|
|

Charles
Deber |
|
|
|
29
May 2008

Ken Lau and Jim Dennis discover
how cell surface receptor levels are regulated by nutrient availability
and by the number of receptor N-glycans
Ken Lau won the Beckman-Coulter Prize for the best
graduate student paper of 2007, and gave a lecture on May 29th at
the International Symposium Celebrating the 100 Anniversary of the
Department of Biochemistry at U. of T.
The Lau et al paper published in Cell
(2007) 129:123 entitled "Complex N-glycan number and degree
of branching cooperate to regulate cell proliferation and differentiation"
provides new insight on the role of protein N-glycosylation in the
adaptation of the cell surface with changes in metabolism.
Their work demonstrates that the number
of N-glycans per polypeptide cooperates with physical properties
of the Golgi pathway to regulate surface levels of receptors and
transporters. Using computational modeling and experimental examples
in T cell and epithelial cells, they show that galectins, a family
of mammalian lectins binds to N-glycan on membrane glycoproteins.
This multivalent interaction enhances surface residency dependent
on the number of N-glycans and their GlcNAc-branching. In turn branching
increases with the availability of the donor substrate UDP-GlcNAc.
UDP-GlcNAc biosynthesis utilizes key metabolites in carbon, nitrogen
and energy homeostasis (fructose-6P, glutamine, acetyl-CoA and UTP).
Glycoproteins with high numbers of N-glycans (e.g. EGFR, IGFR, FGFR,
PDGFR) tend to be bound to galectins, and exhibit early and graded
increase in cell surface expression in responses to increasing UDP-GlcNAc
concentration, while glycoproteins with few N-glycans (e.g. TbR,
CTLA-4, GLUT4) exhibit delayed and switch-like responses. These
results suggest a mechanism for metabolic regulation of cellular
transition between growth and arrest arising from apparent co-evolution
of N-glycan number and biosynthesis. Animal models of reduced N-glycan
branching suggest that this network regulates T cell activation
and autoimmunity, tumor progression, glucose metabolism, and stem
cell maintenance and aging.
Ken completed his PhD in the Dennis
lab and is currently doing postdoctoral work in Dr. Lauffenbergers
group at Harvard.
|
|

Ken
Lau (holding certificate) is joined by (left to right) his supervisor
Jim Dennis, Harry Schachter, who provided advice and support throughout
the project, and Graduate Coordinator, Jim Rini. |
|
|
|
February
2008

Stagljar lab discovers novel
approach to identify pathogen inhibitors
Microbial resistance to antibiotics is a serious and growing threat
to human health. Arnoldo et al. used a novel approach that
combines chemical and genetic perturbation of bakers yeast to find
new targets that might be effective in controlling infections caused
by the opportunistic human pathogen Pseudomonas aeruginosa.
P. aeruginosa is the primary cause of mortality with cystic
fibrosis patients and has demonstrated an alarming ability to resist
antibiotics.
In this study, Arnoldo et al. identified the first small
molecule inhibitors of ExoS, a toxin playing a pivotal role during
P. aeruginosa infections. One of these compounds, exosin,
likely works by modulating the toxin’s enzymatic activity.
The authors further show that this inhibitor protects mammalian
cells against P. aeruginosa infection. Finally, they used
yeast functional genomics tools to identify several yeast homologues
of the known human ExoS targets as possible targets for the toxin.
This innovative approach is an important advance, not only for the
value it may have in cystic fibrosis treatment, but also because
this technique could be used to design novel therapies for any bacterial
pathogen as well as the HIV virus.
In the next phase of their research, the Stagljar lab plans to test
the action of their inhibitors in an animal model of cystic fibrosis.
If successful, the therapeutics may provide an avenue for the treatment
of this debilitating disease.
Arnoldo A, Curak
J, Kittanakom S, Chevelev I, Lee VT, Sahebol-Amri M, Koscik B, Ljuma
L, Roy PJ, Bedalov A, Giaever G, Nislow C, Merrill RA, Lory S, Stagljar
I. Identification of small molecule inhibitors of Pseudomonas
aeruginosa exoenzyme S using a yeast phenotypic screen.
PLoS Genet. (2008) 4(2):e1000005.
|
|

Igor Stagljar
|
|
|
|
30
April 2008

Lewis Kay wins $500,000 Premier's
Discovery Award In Life Sciences and Medicine for his research on
biomolecular NMR
The Premier’s Discovery
Awards celebrate the research excellence of Ontario’s most
accomplished researchers by highlighting their individual achievements
and demonstrating Ontario’s attractiveness as a global research
centre.
Nominees are evaluated on the impact of their work and its contributions
to Ontario’s economy and society, and the extent of their
international recognition.
The following is an excerpt from the
announcement by the Ontario Ministry of Research and Innovation:
Dr. Lewis Kay is a brilliant scientist with an international reputation
in the field of biomolecular nuclear magnetic resonance, a technology
that uses magnetic fields to study the structures of medically important
molecules by measuring how these structures change over time due
to molecular interactions. Dr. Kay is an accomplished biochemist
and has been instrumental in developing new three and four dimensional
nuclear magnetic resonance (NMR) methods. Dr. Kay's work has revolutionized
the field, making it possible for scientists to understand the components
of individual molecules. His discoveries are providing new insights
into how living systems grow and develop. They also improve techniques
for protecting those systems, leading to knowledge about how to
prevent illness. The methods developed by Dr. Kay are in use in
biological NMR laboratories around the world, including labs researching
illnesses such as cancer, diabetes and cardiovascular disease.
|
|

Lewis Kay
|
|
|
 |
| 
|
Research
Highlights from the Past
 |

This section is under construction.
 |
|
|
 |





















